Air density equations Air contains a mixture of dry air and water vapor. The amount of water vapor is a function of the relative humidity; it is also related to the dew point temperature of the air. The addition of water vapor to air reduces the density of the air, which may at first appear counter-intuitive. This occurs because the molar mass of water (18 g/mol) is less than the molar mass of dry air (around 29 g/mol).
For any gas, at a given temperature and pressure, the number of molecules present is constant for a particular volume (see Avogadro's Law). So when water molecules are added to a given volume of air, the dry air molecules must decrease by the same number, to keep the pressure or temperature from increasing. Hence the mass per unit volume of the gas decreases. The density of a substance is the ratio of the mass of a sample of the substance to its volume.
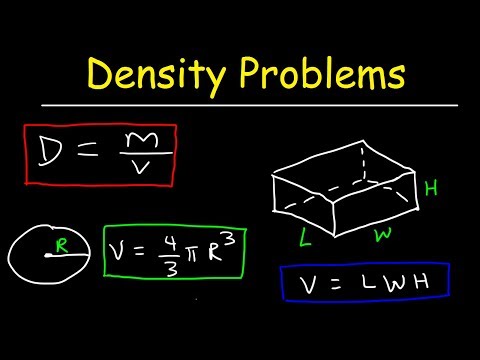
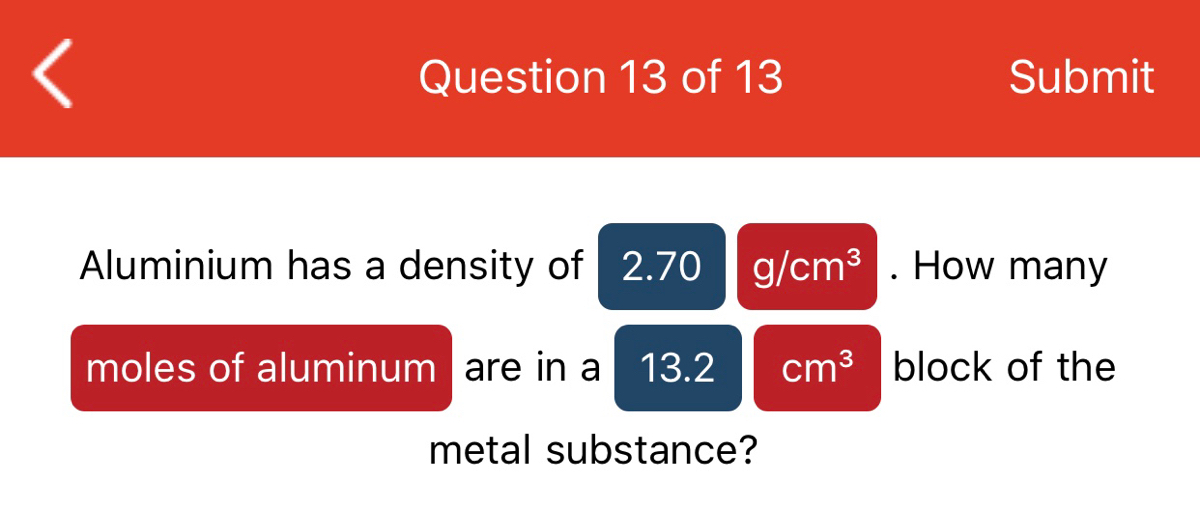
The SI unit for density is the kilogram per cubic meter (kg/m3). For many situations, however, this as an inconvenient unit, and we often use grams per cubic centimeter (g/cm3) for the densities of solids and liquids, and grams per liter (g/L) for gases. Common units for density include g/mL, g/cm3, g/L, or kg/L.
Although there are exceptions, most liquids and solids have densities that range from about 0.7 g/mL to 19 g/mL . Table 1 shows the densities of some common substances. The density of air depends on many factors and can vary in different places. It mainly changes with temperature, relative humidity, pressure and hence with altitude . The air pressure can be related to the weight of the air over a given location. It is easy to imagine that the higher you stand, the less air is above you and the pressure is lower (check out our definition of pressure!).
Therefore, air pressure decreases with increasing altitude. In the following text, you will find out what is the air density at sea level and the standard air density. This section gives you a first insight into the basics of density measurement. You will learn that density is a temperature and pressure-dependent substance property which is often specified with the unit kg/m3 or lb/ft3. The density value is required for determining concentration, average molecular weight and content. For finding the density of gases, it must be noted that this density depends on the respective pressure.
The density of air is the mass per unit of volume it occupies, and it is expressed in kilograms per cubic meter when using the metric system. In the free atmosphere, air density decreases when temperature increases or when the air is humid. However, air density increases when pressure increases. An online air density calculator such as the one by Engineering Toolbox let you calculate theoretical values for air density at given temperatures and pressures. The website also provides an air density table of values at different temperatures and pressures.
These graphs show how density and specific weight decrease at higher values of temperature and pressure. If you need to calculate the density of dry air, you can apply the ideal gas law. This law expresses density as a function of temperature and pressure. Like all gas laws, it is an approximation where real gases are concerned but is very good at low pressures and temperatures.
Increasing temperature and pressure adds error to the calculation. For a better understanding of how temperature and pressure influence air density, let's focus on a case of dry air. It contains mostly molecules of nitrogen and oxygen that are moving around at incredible speeds. Use our particles velocity calculator to see how fast they can move!
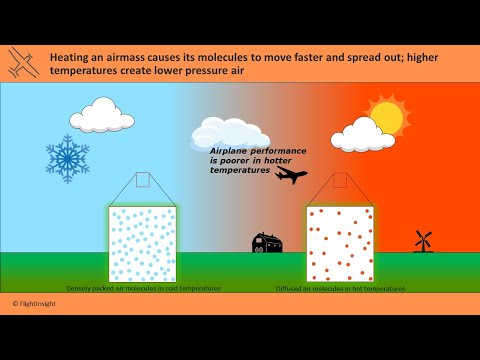
For example, the average speed of a nitrogen molecule with a mass of 14 u (u - unified atomic mass unit) at room temperature is about 670 m/s - two times faster than the speed of sound! Moreover, at higher temperatures, gas molecules further accelerate. As a result, they push harder against their surroundings, expanding the volume of the gas .
And the higher the volume with the same amount of particles, the lower the density. Therefore, air's density decreases as the air is heated. For any ideal gas, at a given temperature and pressure, the number of molecules is constant for a particular volume (see Avogadro's Law). Gas specific gravity is defined as the ratio of the density of the gas to the density of air at 1atm pressure at 60°F . If ideal gas law behavior is assumed, gas specific gravity is the molecular weight of the gas divided by the molecular weight of air. Because natural gas is a mixture, the molecular weight used is a weighted average molecular weight based on the components of the gas.
The earth's atmosphere exerts a pressure, as does any other gas. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects . Although the force of each collision is very small, any surface of appreciable area experiences a large number of collisions in a short time, which can result in a high pressure.
In fact, normal air pressure is strong enough to crush a metal container when not balanced by equal pressure from inside the container. In practical terms, density is the weight of a substance for a specific volume. The density of water is roughly 1 gram per milliliter but, this changes with temperature or if there are substances dissolved in it.
Ice is less dense than liquid water which is why your ice cubes float in your glass. As you might expect, water density is an important water measurement. The density of moist air is calculated as the sum of the density of the dry air component of the mixture plus the density of the saturated component of the mixture. The water vapour pressure is then subtracted from the total pressure to give the pressure of the dry component of the parcel. Densities of the two components are then calculated and summed to give the final answer.
The density of air is the mass per unit volume of Earth's atmosphere. Air density, like air pressure, decreases with increasing altitude. It also changes with variation in temperature or humidity. At sea level and at 15 °C, air has a density of approximately 1.225 kg/m3 according to ISA . At sea level and at 15 C, air has a density of approximately 1.225 kg/m3 according to ISA (International...
The density of air is usually denoted by the Greek letter ρ, and it measures the mass of air per unit volume (e.g. g / m3). Dry air mostly consists of nitrogen (~78 %) and oxygen (~21 %). The remaining 1 % contains many different gases, among others, argon, carbon dioxide, neon or helium. However, the air will cease to be dry air when water vapor appears.
The calculator below can be used to calculate the air density and specific weight at given temperatures and atmospheric pressure. A hydrometer is used to measure density of a liquid.The instrument to measure the density of a liquid is called a hydrometer. It is one of the simplest of scientific-measuring devices, and you can even make your own out of a plastic straws . More often, though, it is made of glass and looks a lot like a thermometer. It consists of a cylindrical stem and a weighted bulb at the bottom to make it float upright. The hydrometer is gently lowered into the liquid to be measured until the hydrometer floats freely.
There are etched or marked lines on the device so the user can see how high or low the hydrometer is floating. In less dense liquids the hydrometer will float lower, while in more dense liquids it will float higher. Since water is the "standard" by which other liquids are measured, the mark for water is probably labeled as "1.000"; hence, the specific gravity of water at about 4°C is 1.000. The reason is that the divisor in every case is 1 gram per cubic centimeter, the density of water.
The only difference between density and specific gravity for solids and liquids is that specific gravity has no label. In dividing 7.87 grams per cubic centimeter by 1.00 gram per cubic centimeter, the labels divide out , leaving only the number. In the following example, the mass is found directly by weighing, but the volume is found indirectly through length measurements.
Because the density of water in g/cm3 is 1.0, the SG of an object is will be almost the same as its density in g/cm3. However, specific gravity is a unitless number, and is the same in the metric system or any other measurement system. It is very useful when comparing the density of two objects. Since specific gravity is unitless, it doesn't matter whether the density was measured in g/cm3 or in some other units (like lbs/ft3).
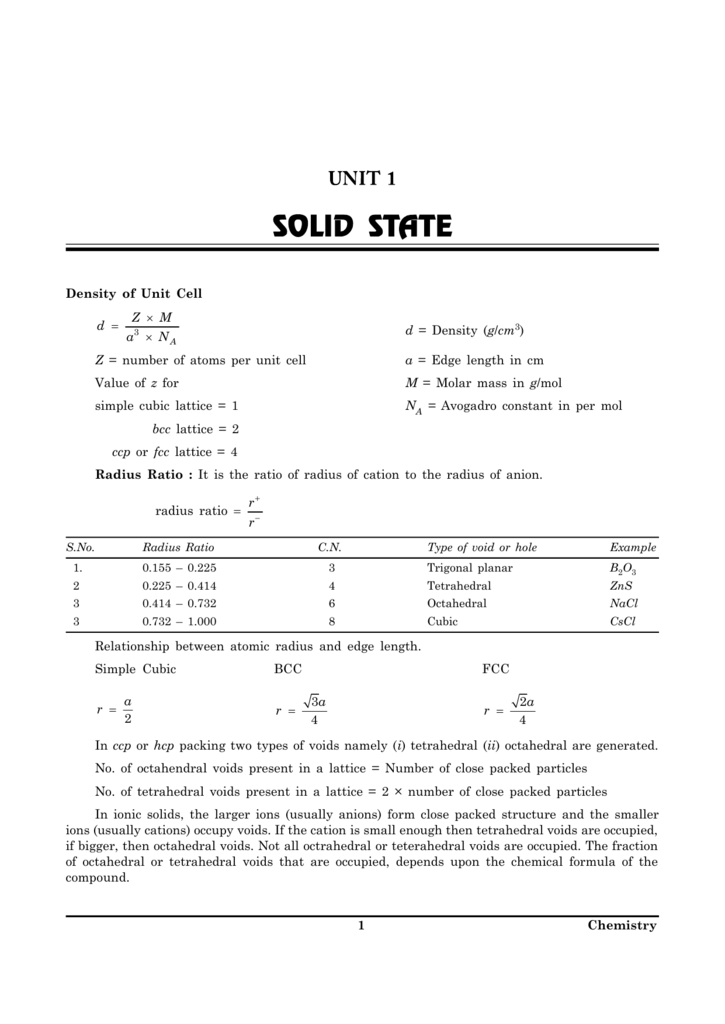
A new formulation of the density of air-saturated water as a function of temperature on the 1990 International Temperature Scale (ITS-90) is presented. Also, a new equation for calculating isothermal compressibility as a function of temperature on ITS-90 was developed. The equations are to be used to calculate the density of water, in the temperature range 5 to 40 °C on ITS-90, used in the gravimetric determination of the volume of volumetric standards.
When the substance is a solid at 25º, determine the specific gravity of the melted material at the temperature directed in the individual monograph, and refer towaterat 25º. The idea of integrating along the particle's path is daunting. The pressure here at the surface of the earth, although partly due to dynamic effects of air movement, is mostly due to the total of all the weight of the air above that point. Force is the total weight of all the air stacked above. Weight is mass times the acceleration due to gravity. The cross-sectional area of a column of air radiating directly upward, gets larger as it rises.
The acceleration of gravity decreases as you get farther away. However, the earth is so large and the atmosphere so thin, that both of these values are essentially constant (to within 1%). In the gravimetric determination of the volume of volumetric standards, water is used as the calibrating fluid.
The volume is calculated from the mass and density of the water. In many quarters, the formulation of Wagenbreth and Blanke is used to calculate the density of water. In this paper, a new formulation of the density of water (based primarily on the work of Kell ) as a function of temperature on the 1990 International Temperature Scale is presented.
True density is the ratio between the mass and volume of a substance at a given pressure and temperature, corresponding to its weight in a vacuum. This is the concept also used in density measurement by digital density meters. The density of air, is the mass per unit volume of Earth's atmosphere. The density of air is the mass per unit volume of atmospheric gases. The density of air, or how light it is, depends on the temperature and pressure of the air.
Typically, the value given for the density of air is at STP . A well-known approximation of dew point is a logarithmic function of relative humidity. As you may know, when the function of a logarithm approaches zero, its value goes to minus infinity.
Therefore, a dew point doesn't exist for the zero relative humidity. However, you can still calculate what is the density of dry air with our air density calculator! Just select dry air in the "air type" field, where we have ignored dew point/relative humidity in the computations. A cold dry air mass has a greater density than a more humid air mass. This would mean that the humid air mass has lower air pressure. This is because the molecular weight of water is less than the molecular weight of dry air.
What two things do you need to know in order to find the density of water? Students should realize that they need both the volume and mass of a sample of water to find its density. Suggest that students use a graduated cylinder to measure volume in milliliters. Suggest that students use a balance to measure the mass in grams. Tell students that they can find mass by weighing the water.
However, since water is a liquid, it needs to be in some sort of container. So in order to weigh the water, they have to weigh the container, too. Explain to students that they will have to subtract the mass of an empty graduated cylinder from the mass of the cylinder and water to get the mass of just the water. The difference is the bouyancy force of the air that is displaced. Density is defined as mass/volume, but for practical reasons this is often substituted by weight/volume.
The weight is measured by measuring the force with which the substance is attracted to earth by gravity. The net force is affected by the bouyancy of the surrounding fluid. This results in the weight being less if it is measured in water than if it is measured in air, and similarly it weighs less when measured in air than in vacuum. We can measure atmospheric pressure, the force exerted by the atmosphere on the earth's surface, with a barometer . A barometer is a glass tube that is closed at one end, filled with a nonvolatile liquid such as mercury, and then inverted and immersed in a container of that liquid.
The atmosphere exerts pressure on the liquid outside the tube, the column of liquid exerts pressure inside the tube, and the pressure at the liquid surface is the same inside and outside the tube. The height of the liquid in the tube is therefore proportional to the pressure exerted by the atmosphere. To get a better idea of the density of air specifically, you need to account for how air is made of different gases when formulating its density. At a constant temperature, pressure and volume, dry air is typically made of 78% nitrogen (N2), 21% oxygen (O2) and one percent argon (Ar). For example, the air is less dense in Denver than in Miami.

























No comments:
Post a Comment
Note: Only a member of this blog may post a comment.